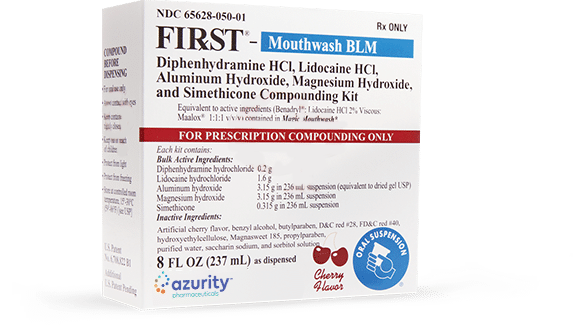
- Product Information
Rx Only | For Prescription Compounding Only
Two sizes available:
- 4 OZ (119 mL) as dispensed
- 8 OZ (237 mL) as dispensed
Equivalent to active ingredients (Benadryl®:
Lidocaine HCl 2% Viscous: Maalox® 1:1:1 v/v/v) contained in Magic Mouthwash** This product is not manufactured or distributed by Johnson & Johnson, a subsidiary of McNeil Consumer Healthcare, manufacturer of Benadryl® or by Novartis Consumer Health, Inc. manufacturer of Maalox®.
- Ordering Information
NDC # Wholesaler FIRST® – Mouthwash BLM
4 oz.
65628-0050-04FIRST® – Mouthwash BLM
8 oz.
65628-0050-01Website ABC 10101664 10003880 www.amerisourcebergen.com ANDA 601403 610485 www.andanet.com Cardinal Health 4541777 3619210 www.cardinal.com Dakota Drug — 648725 www.dakdrug.com Louisiana Wholesale Drug — — www.lwdrx.com McKesson Drug 2191484 1287119 www.mckesson.com Morris & Dickson 266064 628750 www.morrisdickson.com Mutual Drug — 456459 www.mutualdrug.com Smith Drug 397992 577858 www.smithdrug.com Value Drug 926386 392324 www.valuedrugco.com - Ingredients
FIRST® – Mouthwash BLM
4oz. 118 mLFIRST® – Mouthwash BLM
8 oz. 236 mLActive Ingredients Diphenhydramine hydrochloride 0.1 g 0.2 g Lidocaine hydrochloride 0.8 g 1.6 g Aluminum hydroxide 1.58 g 3.15 g Magnesium hydroxide 1.58 g 3.15 g Simethicone 0.158 g 0.315 g Inactive Ingredients Benzyl alcohol, butylparaben, cherry flavor, D&C red #28, FD&C red #40, hydroxyethylcellulose, Magnasweet 185, propylparaben, purified water, saccharin sodium, and sorbitol solution.
To report SUSPECTED ADVERSE REACTIONS, contact Azurity Pharmaceuticals at 1-800-461-7449, or FDA at 1-800-FDA-1088 or www.fda.gov/MedWatch